Understanding the Properties and Uses of Oxide Acids: A Comprehensive Guide
Oxide Acid:
When we speak of acids, most people tend to think of the more common acids such as hydrochloric acid or sulfuric acid. However, there is another category of acids – oxide acids. Oxide acids are compounds that have both oxygen and hydrogen atoms combined with a non-metal element, with metal atoms present in the chemical formula as well. This article will take a closer look at what oxide acids are, how they form, and some of their characteristics.
What are Oxide Acids?
Oxide acids can be defined as binary or ternary inorganic compounds that contain hydrogen, oxygen, and one or more non-metals. These acids play important roles in a variety of chemical reactions, including the formation of sulfuric acid and nitric acid.
How are Oxide Acids Formed?
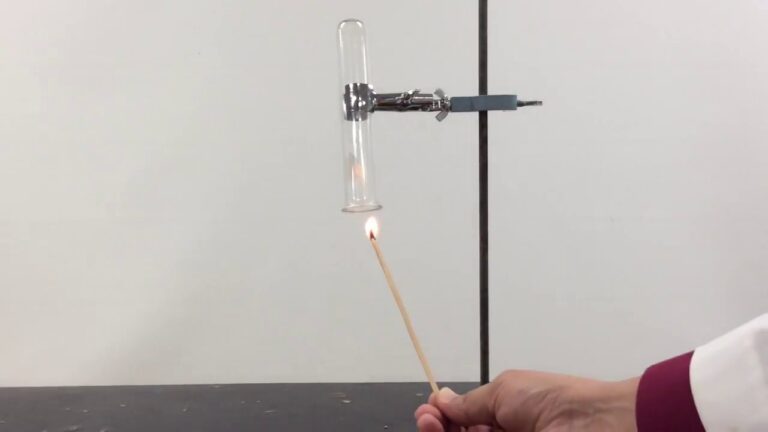
Oxide acids are formed through a process called oxidation. Oxidation is a chemical reaction that involves the loss of one or more electrons from an atom or molecule. In the case of oxide acids, oxygen atoms combine with hydrogen atoms and one or more non-metal atoms to form an acidic compound.
Some examples of oxide acids include:
- Sulfuric acid (H2SO4) – This is one of the most commonly used industrial acids in the world. Sulfuric acid is widely used in the production of fertilizers, detergents, and other chemicals.
- Nitric acid (HNO3) – This is another heavily used industrial acid. Nitric acid is used in the production of fertilizers, dyes, and explosives.
- Phosphoric acid (H3PO4) – This acid is commonly used as a rust remover and is also a key ingredient in many soft drinks.
The Characteristics of Oxide Acids
There are several characteristics of oxide acids that make them unique:
- Acidity: Oxide acids are known for their strong acidic properties.
- Corrosiveness: Oxide acids can be highly corrosive and can cause damage to metals and other materials.
- Polarity: Oxide acids tend to be highly polar due to the presence of multiple oxygen atoms in their chemical makeup.
Conclusion
Oxide acids may not be as well-known as other types of acids, but they play an important role in many industrial processes. Being aware of the characteristics of these acids can help us understand their many uses and applications in various fields.
Contents
Most searched products:
Does Sephora Support Israel? Answering Your Questions
The Ultimate Guide to Azealic Acid: Benefits, Uses, and Side Effects
How Long Does Glycolic Acid Take to Show Results: Your Ultimate Guide
Discover the Benefits of The Ordinary Botox for Your Skin
The Ultimate Reviews of The Ordinary Peeling Solution
The Ultimate Guide to The Ordinary Colours Foundation: Reviews, Swatches, and Tips
The Perfect Order: When to Use Retinol and Niacinamide in Your Skincare Routine
Unlock Smooth and Supple Skin: Discover the Best Skincare Products for Skin Suppleness
Say Goodbye to B.O with Glycolic Acid Deodorant: The Secret to Long-Lasting Freshness
Exploring the Wonders of The Ordinary Oxford Street: A Complete Guide