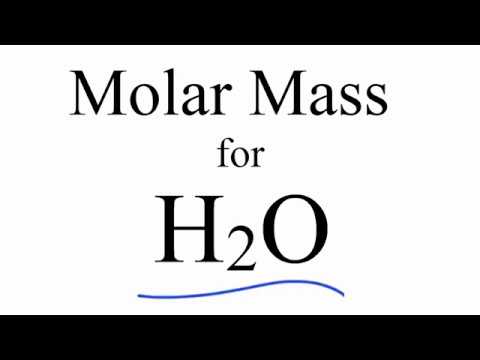Understanding the Importance of Water’s pKa Value for Chemical Reactions
Water is a ubiquitous substance in our daily lives, composing up to 60% of our body weight and covering over 70% of our planet’s surface. One aspect that is less commonly known about water is its acidity level, or pKa. The pKa of water is an important factor in understanding the chemical reactions involving water and its impact on various biological and environmental systems. In this article, we will discuss the pKa of water and its implications.
Firstly, let us define what pKa means. It is a measure of acidity or basicity, expressed as the negative logarithm of the acid dissociation constant (Ka). The pKa value of a substance indicates the degree to which it can donate or accept protons, with lower pKa indicating stronger acidity and higher pKa indicating stronger basicity. The pKa of water is 15.7, indicating that it is a very weak acid.
One application of knowing the pKa of water is in the study of acid-base equilibrium. In a neutral solution, the concentration of hydrogen ions (H+) and hydroxide ions (OH-) are equal, with a pH of 7. However, when an acid or base is introduced, the concentration of ions will shift, depending on the pKa of the substance. For example, a strong acid will readily donate a proton to water, decreasing the pH of the solution. In contrast, a weak acid like water will only donate protons when in the presence of a stronger acid.
Another area where the pKa of water is relevant is in the field of biochemistry. The human body functions within a narrow range of pH values, and any deviation from this range can lead to health complications. The pKa of water plays a role in buffering the pH in the body’s fluids and preventing it from becoming too acidic or too basic. The pH of blood, for example, is maintained at around 7.4, which is slightly basic. If the pH were to drop below 7.35, it could lead to acidosis, a condition where the body’s fluids become too acidic and disrupt various metabolic processes.
The pKa of water also has implications for environmental systems, such as aquatic ecosystems. The pH of water bodies can be influenced by various factors, such as rainfall, pollution, and geological features. Any changes in pH can have a significant impact on the aquatic life that relies on that ecosystem. For instance, if the pH of a freshwater stream drops below 5.5, it can be lethal to most aquatic organisms.
In conclusion, the pKa of water is a crucial factor in understanding the chemical properties and reactions of water. Its weak acidic nature and neutral pH make it an essential component in many biological and environmental systems. Understanding the pKa of water can also help us grasp the impact of changing pH levels in different contexts, from biochemistry to aquatic ecosystems.
Most searched products:
5 Surprising Ways The Ordinary Lash Serum Can Transform Your Lashes
Is Niacinamide an Active Ingredient in Skincare Products?
Get Perfect Skin with Our Comprehensive Skin Care Package
Discover the Best Cleansing Balm at Superdrug for Flawless Skin
Does Sephora Support Israel? Answering Your Questions
10 Best Skin Clarifying Masks for a Clear and Glowing Complexion
The Ultimate Guide to Using The Ordinary Hydrochloric Acid: Benefits and Precautions
The Ultimate Guide to Choosing and Using the Best Hydrolic Acid Serum
Maximizing the Benefits: How Long Should You Leave Salicylic Acid on Your Face?
Transform Your Skin with Our Effective and Moisturizing Hydrating Cream