The Ultimate Guide to Understanding Acid Formulas for Beginners
Acid Formula: Understanding the Essentials
If you’re reading this then you’re likely familiar with acids – those substances that are capable of donating a hydrogen ion (H+) to a solution or compound. Acids are used in a variety of applications, from cleaning products to the medical field. But how are acids actually represented and what goes into their formulas? In this article, we’ll take a deep dive into the basics of acid formula and all that comes with it.
What is an Acid Formula?
An acid formula is a way to represent the composition of an acid using chemical symbols and numbers. It shows how many atoms of each element make up the acid, as well as how they are bonded together. The general formula for an acid is written as HX, where X is an anion.
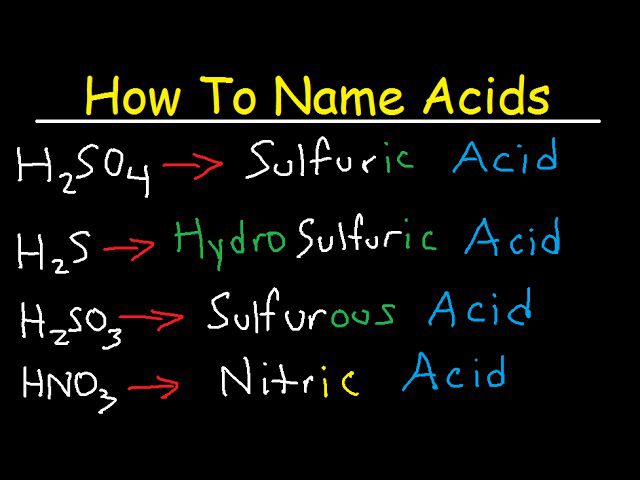
The Naming Convention of Acids
Before we delve into the formula, however, it’s important to first understand how acids are named. The name of an acid is determined by the anion it contains. For anions that end in -ide, the acid name starts with “hydro-” and ends with “ic,” for example, hydrochloric acid (HCl). For anions that end in -ate or -ite, the acid name changes the suffix to -ic or -ous respectively. For example, sulfuric acid (H2SO4) and sulfurous acid (H2SO3) are both derived from the same anion, SO42-.
Deconstructing an Acid Formula
Now, let’s break down an acid formula. In its simplest form, HX, hydrogen (H) is bonded to an anion (X). The anion itself can be made up of any number of elements, but it’s important to note that only one hydrogen is typically present in an acid.
Polyprotic Acids
It’s also worth mentioning the existence of polyprotic acids – acids that contain more than one hydrogen ion that can be donated. In their formula, the number of hydrogen ions is represented by a subscript. For example, sulfuric acid (H2SO4) can donate two hydrogen ions, hence the subscript “2”.
Acid Strength
Lastly, we come to acid strength. The strength of an acid is determined by how easily it can donate a hydrogen ion to another compound or solution. Strong acids, such as hydrochloric acid and sulfuric acid, are able to donate hydrogen ions easily, while weak acids, such as acetic acid (CH3COOH), do so less readily.
Conclusion
In summary, an acid formula is a way to represent the composition of the acid, with hydrogen bonded to an anion in the simplest form of HX. Acids are named based on their anion, with polyprotic acids containing more than one hydrogen ion. The strength of an acid is determined by how easily it can donate a hydrogen ion. Understanding acid formula is key to understanding the role of acids in our everyday lives, from the products we use for cleaning to the food we eat.
Most searched products:
Does Sephora Support Israel? Answering Your Questions
The Ultimate Guide to Azealic Acid: Benefits, Uses, and Side Effects
How Long Does Glycolic Acid Take to Show Results: Your Ultimate Guide
Discover the Benefits of The Ordinary Botox for Your Skin
The Ultimate Reviews of The Ordinary Peeling Solution
The Ultimate Guide to The Ordinary Colours Foundation: Reviews, Swatches, and Tips
The Perfect Order: When to Use Retinol and Niacinamide in Your Skincare Routine
Unlock Smooth and Supple Skin: Discover the Best Skincare Products for Skin Suppleness
Say Goodbye to B.O with Glycolic Acid Deodorant: The Secret to Long-Lasting Freshness
Exploring the Wonders of The Ordinary Oxford Street: A Complete Guide