Mastering the Sodium Formula: A Comprehensive Guide to Understanding Sodium Compounds
Sodium Formula: Everything You Need to Know
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal that is an essential component of many chemicals, including common table salt. Sodium plays a critical role in many biological processes, and its chemical formula is crucial to understanding its properties.
The Chemical Formula for Sodium
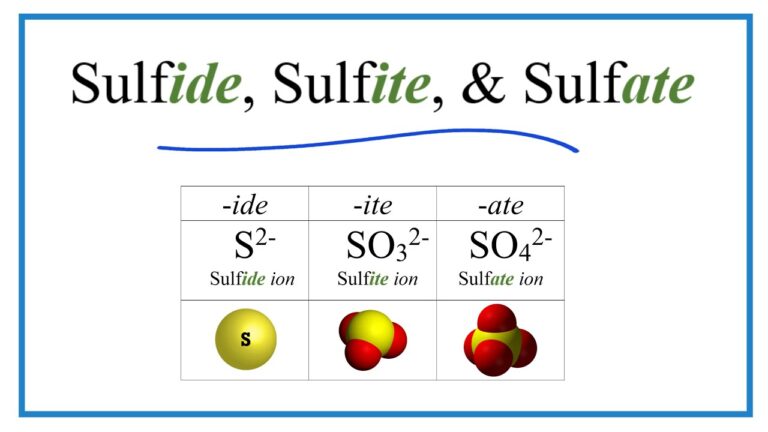
The chemical formula for sodium is Na. This simple formula represents one atom of sodium, which has 11 protons, in its atomic nucleus. When combined with other elements or compounds, sodium can form a wide variety of chemical formulas. For example, when sodium reacts with chlorine, it forms table salt or Sodium Chloride (NaCl). Similarly, sodium can react with other elements such as oxygen (forming Sodium Oxide; Na2O) and sulfur (forming Sodium Sulphide; Na2S).
The Properties of Sodium
Sodium is a highly reactive metal that can catch fire when it comes into contact with water or air. It is so reactive that it is never found in its pure, metallic form in nature. Instead, it is usually found as a compound, such as table salt or baking soda (Sodium Bicarbonate; NaHCO3). Sodium has a low melting point of 97.72°C (208.9°F) and a boiling point of 882.94°C (1,611.3°F). It is a soft metal and has a density of 0.97 grams per cubic centimeter.
Uses and Applications of Sodium
Sodium has a wide variety of uses and applications. Because of its high reactivity, it is a key component in many chemical reactions. Sodium is widely used in the production of chemicals such as sodium hydroxide (NaOH), sodium carbonate (Na2CO3), and its derivatives. Sodium is also used in the production of pulp and paper, textiles, and detergents. In addition, Sodium Chloride (NaCl), which is the most common compound of sodium, is widely used in cooking and food preservation.
The Dangers of Sodium
While sodium is an essential element for human health, consuming too much sodium can be dangerous. Excess sodium intake has been linked to high blood pressure, heart disease, and stroke. Therefore, experts recommend limiting sodium intake to 2,300 milligrams per day for most adults. Sodium can also be hazardous to the environment, especially when improperly disposed of. Sodium can contaminate soil and water sources, which can have long-lasting effects on the ecosystem.
Conclusion
In conclusion, sodium plays a crucial role in many biological and chemical processes. Its chemical formula, Na, is a fundamental concept to understanding its properties and uses. While it can be hazardous to health and the environment in excess, it remains an essential element for many industries and applications. Whether you are a chemist, a cook, or simply someone who eats, it is essential to understand the basic properties and uses of this vital element.
Most searched products:
Does Sephora Support Israel? Answering Your Questions
The Ultimate Guide to Azealic Acid: Benefits, Uses, and Side Effects
How Long Does Glycolic Acid Take to Show Results: Your Ultimate Guide
Discover the Benefits of The Ordinary Botox for Your Skin
The Ultimate Reviews of The Ordinary Peeling Solution
The Ultimate Guide to The Ordinary Colours Foundation: Reviews, Swatches, and Tips
The Perfect Order: When to Use Retinol and Niacinamide in Your Skincare Routine
Unlock Smooth and Supple Skin: Discover the Best Skincare Products for Skin Suppleness
Say Goodbye to B.O with Glycolic Acid Deodorant: The Secret to Long-Lasting Freshness
Exploring the Wonders of The Ordinary Oxford Street: A Complete Guide