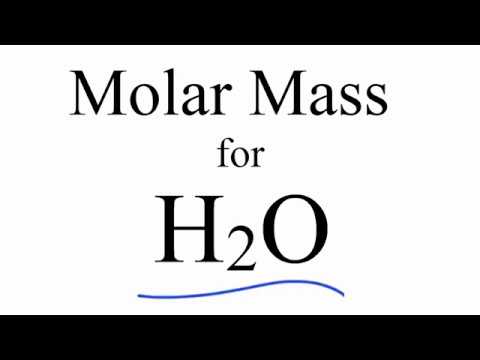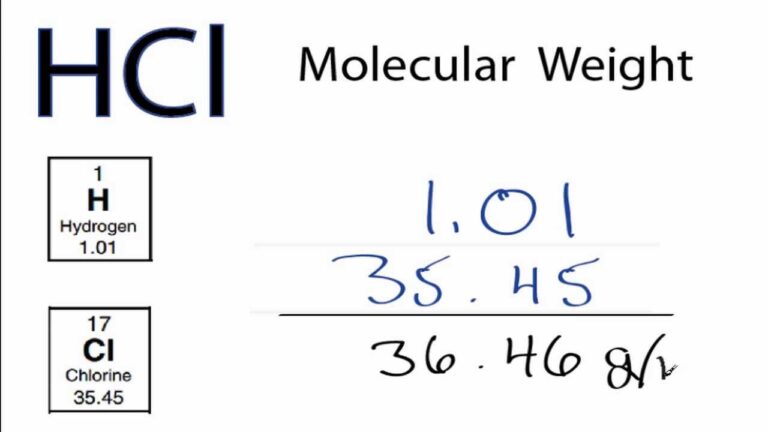Exploring the Molar Mass of Zinc: Formula and Significance
Zinc is a mineral necessary for our bodies to function properly. It is found in foods such as meat, dairy, and whole grains. However, sometimes we need to supplement our diets with additional zinc. In order to do this, we need to know the molar mass of zinc.
Molar mass of zinc refers to the mass of one mole of a zinc element. A mole is a unit of measurement used in chemistry and it represents a certain number of particles, in this case, atoms. The molar mass of zinc is 65.38 g/mol.
Knowing the molar mass of zinc is important in various chemical reactions. It allows chemists to accurately measure how much zinc is needed to react with other chemicals, as well as to determine the purity of a zinc compound.
Zinc has many uses, including in the production of galvanized steel which is used in building construction and automobile manufacturing. It is also used in the production of zinc oxide which is used in the manufacturing of rubber, cement, and paint. Additionally, zinc is important for maintaining a healthy immune system and it is a common ingredient in many dietary supplements.
If you are wondering how to calculate the molar mass of zinc, it’s relatively simple. First, you need to determine the atomic mass of zinc, which can be found on the periodic table. Zinc has an atomic mass of 65.38 g/mol. This means that one mole of zinc atoms weighs 65.38 grams.
To calculate the molar mass of a zinc compound, you need to determine how many atoms of each element are present in the compound and multiply that by the atomic mass of each element. For example, in the compound ZnCl2 (zinc chloride), you have one zinc atom with a mass of 65.38 g/mol and two chlorine atoms with a mass of 35.45 g/mol each.
To calculate the molar mass of ZnCl2, you would add the atomic masses of each element:
(1 x 65.38 g/mol) + (2 x 35.45 g/mol) = 136.28 g/mol
Therefore, the molar mass of ZnCl2 is 136.28 g/mol.
In conclusion, understanding the molar mass of zinc is important for various chemical applications as well as for maintaining a healthy immune system through dietary supplements. Knowing the molar mass of zinc will allow for accurate measurement and calculation in chemical reactions involving zinc compounds.
Most searched products:
Is Niacinamide an Active Ingredient in Skincare Products?
5 Surprising Ways The Ordinary Lash Serum Can Transform Your Lashes
Top 10 Exfoliators for Smooth and Glowing Skin
Saylic Acid: The Ultimate Guide for Clear Skin
Egg Cleansing Results: A Comprehensive Guide to the Benefits and Effectiveness
Why The Ordinary Glycolic Acid Is Perfect For Banishing Armpit Darkness
Revitalize Your Skin After a Sweat Session with the Best Post-Workout Skincare Products
The Ultimate Guide to The Ordinary Eye Cream: Reviews, Ingredients, and Benefits
Discover the Benefits of The Ordinary Botox for Your Skin
Benefits of Using Glycolic Acid for Dandruff Treatment